
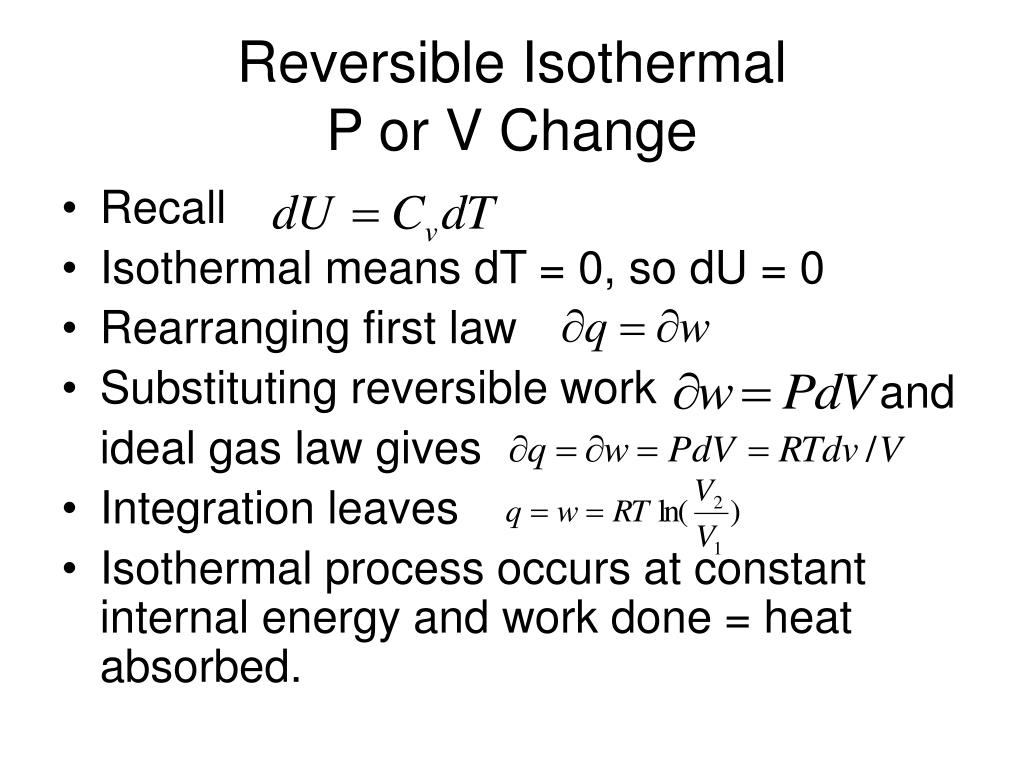
In Italian textbooks, the law is called the first Gay-Lussac law. Some textbooks in English call it Charles’s and Gay-Lussac law.
Because Charles did not publish the results of his experiments, the law of volumes is occasionally called Gay-Lussac law. A French chemist and physicist Louis Gay-Lussac referenced this experiment 15 years later in his paper on the relationship between the volume and temperature of gases. His measurements showed that the volume of different gases increase is proportional to the temperature increase. This empirical law was first formulated by a French scientist Jacques Charles in 1787 while studying the buoyancy of balloons filled with different gases in the air. For example, to find the initial volume V 1, we will write Any unknown term of this proportion can be found by means of cross-multiplication. Where V is the volume, T is the absolute temperature and the subscripts 1 and 2 refer to the initial and final states of a gas in a system. Or, if we consider the pairing of variables at different states (initial and final) of the same process, we can write a proportion: Mathematically, this relationship can be written as This law is also known as the law of volumes. The law states that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature if its pressure remains constant. The picture is in the public domainĬharles’s law is an experimental law that describes how ideal gas expands when heated. Behind the balloon is the Tuileries Palace that was destroyed by the fire in 1871. determine the work output from each engine.The first flight of a manned hydrogen-filled balloon piloted by Jacques Charles and Nicolas-Louis Robert in 1783.
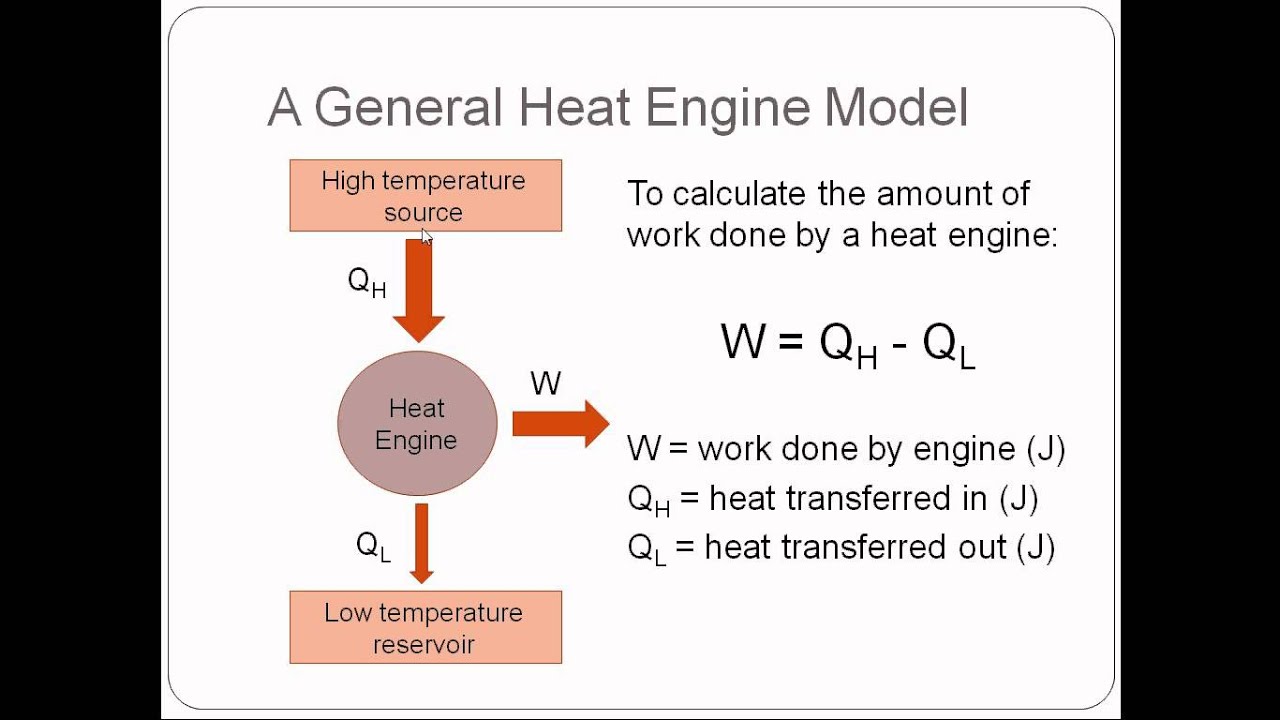
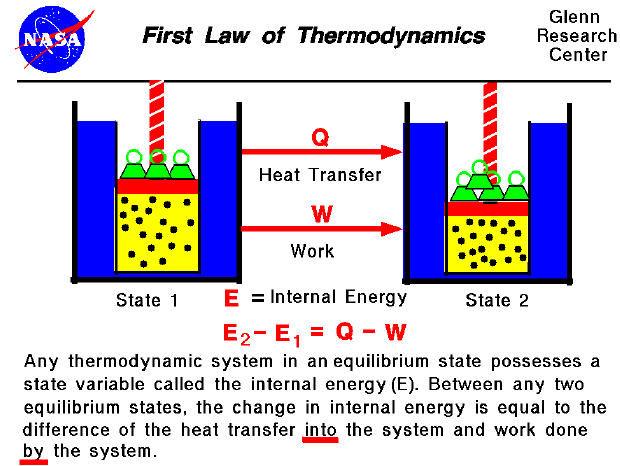
The first engine absorbs 2400 kJ of heat from a thermal reservoir at 1250 K and the third engine ejects its waste of 300 kJ to a sink at 150 K. Three real heat engines have the same thermal efficiency and are connected in series.Calculate the maximum efficiency of an engine operating between 120 o C and 35 o C.Define Kelvin Planck and Clausius statements of the second law of Thermodynamics.Find the source and the sink temperature. If the sink temperature is reduced by 20 o C, the efficiency is increased to 30%. Carnot engine working between a source temperature of T 2 and sink temperature of T 1 has efficiency of 25%.If the sink temperature is reduced by 62 o C, the efficiency gets doubled. A Carnot engine converts one sixth of the heat input into work.A Carnot engine has the same efficiency (i) between 100 K and 500 K and (ii) between T K and 900 K.Define Carnot Theorem and also give its proof.Also give the consequences of the Carnot cycle. If the sink temperature is reduced by 80 o C, the efficiency gets doubled. A Carnot engine converts one fifth of the heat input into work.the net work done by the system.Ĭombining this with equation (1), we will get According to the first law of thermodynamics, the work obtained is equal to the difference between the heat supplied by the source (q 2) and the heat rejected to the sink (q 1 i.e. The work delivered from the system during the cycle is represented by the enclosed area of the cycle. the difference between the temperature of the source and the sink, greater will be the efficiency. No heat engine can have efficiency equal to one.įrom above equation we can conclude that greater the difference between the temperature T 2 – and T 1 i.e. less than 1, the efficiency of a heat engine is always less than 1. (1)Īs (T 2 – T 1) / T 2 is infinitesimally small i.e. The fraction of the heat absorbed by an engine which can be converted into work is known as efficiency of the heat engine.Įfficiency, η = w / q 2 = (T 2 – T 1) / T 2 …………….


 0 kommentar(er)
0 kommentar(er)
